Introduction
Chimeric antigen receptor (CAR) T-cell therapy provides long-term remissions in a substantial proportion of patients with large B-cell lymphoma (LBCL) who have relapsed or are refractory (R/R) to chemoimmunotherapy. The identification of prognostic factors to identify which patients will benefit most from this therapy is crucial to improve patient selection. Even though metabolic tumor burden assessed by 18F-fluorodeoxyglucose Positron Emission Tomography (PET) has a confirmed prognostic value in the setting of chemoimmunotherapy, its predictive role after CAR T-cell therapy is not established.
Methods
We conducted a single-center study including all patients with R/R LBCL who received a single infusion of CD19-targeted second-generation CAR T-cells carrying a 4-1BB costimulatory domain from July 2018 to January 2020. Adverse events were graded according to the American Society for Transplantation and Cellular Therapy (ASTCT) consensus. All patients underwent a baseline PET scan and metabolic disease evaluation after infusion. Disease response assessment was conducted according to Lugano criteria. Metabolic tumor volume (MTV) and maximum standardized uptake value (SUVmax) were measured at baseline and at 1-month after CAR T-cell infusion, and correlated with disease response and development of adverse events. To identify the optimal cut-offs for metabolic parameters we used the maximally selected log-rank statistics in the PFS analysis.
Results
Thirty-five consecutive patients with R/R LBCL who received CAR T-cell therapy were included in the study. Patients' baseline characteristics are summarized in Table 1. Median age at treatment was 58 years, and 74% were males. At the time of diagnosis most of them had an advanced stage of disease (86%) and were refractory to the last therapy (n=31, 88%).
Best response after CAR T-cell therapy included 9 (26%) patients in complete remission (CR) and 16 (46%) in partial remission (PR). Ten (28%) patients were in progressive disease (PD) at the 1-month disease evaluation. At a median follow-up of 7.6 months, median PFS and OS were 3.4 months and 8.2 months, respectively. Regarding toxicity, eleven (31%) patients developed clinically significant CAR T-cell related toxicity, defined as grade 2 or higher CRS (n=7, 20%) and grade 2 or higher ICANS (n=6, 17%).
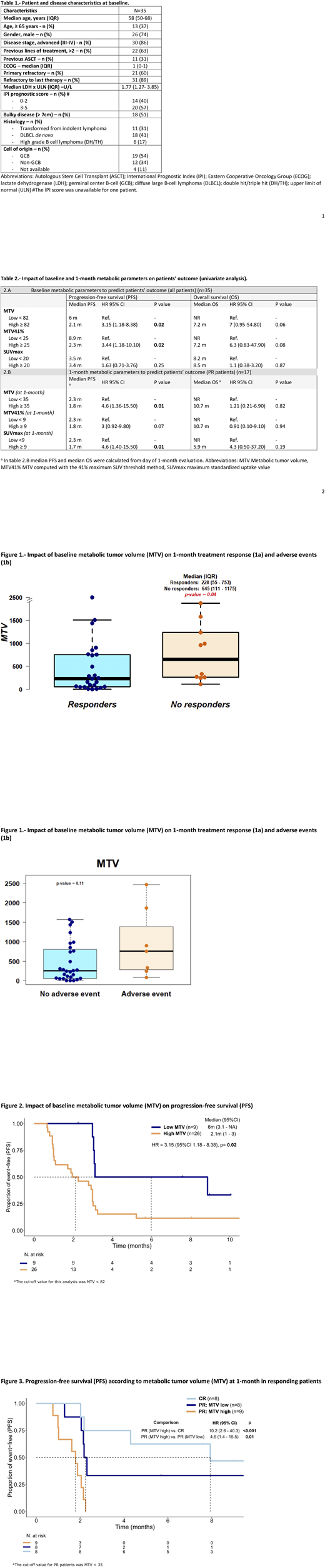
Median baseline MTV and MTV41% were 270 cm3(IQR 87-875) and 119 cm3 (IQR 32-300), respectively. Median SUVmax was 24 (IQR 17-32). Patients who responded (CR and PR) had lower baseline MTV values compared with non-responders (median of 228 cm3 vs 645 cm3, p=0.04) (Figure 1a). No association was found between MTV41% or SUVmax and disease response. In terms of PFS, a high baseline MTV (>82 cm3) was associated with a lower PFS compared to patients with lower MTV values (median PFS, 2.1 months vs. 6 months; HR 3.15, p= 0.02) (Table 2 and Figure 2). Patients with high baseline MTV41% values (>25 cm3) also had an inferior PFS (HR 3.44, p= 0.02); no association was found between baseline SUVmax and PFS (Table 2). As per OS, there was no significant association with baseline MTV, MTV41% and SUVmax (Table 2).
Regarding toxicity, there was no significant association between baseline MTV, MTV 41% and SUVmax values with grade 2 or higher CRS and ICANS events (Figure 1b).
All patients underwent a 1-month post-infusion PET evaluation. Disease response at this timepoint was: CR in 8 patients (23%), PR in 17 patients (49%) and PD in 10 patients (28%). For patients in CR and PR at 1-month the probability of PFS at 6 months was 62.5% and 12.7%, respectively (HR=3.89, p=0.02). For patients in PR at the 1-month evaluation, MTV values at that timepoint were predictive for PFS; patients in PR with low (<35 cm3) and high 1-month MTV values had a 6m-PFS of 33% and 0%, respectively (Figure 3)(HR=4.6, p = 0.01). In these patients, SUVmax values also predicted PFS and a trend towards significance was observed for MTV41% (HR=3, p=0.07).
Conclusion
Metabolic tumor burden parameters measured by 18FDG-PET before and 1-month after CAR-T cell infusion identify LBCL patients who benefit most from these therapies and could aid patient selection.
Iacoboni:Novartis, Gilead, Celgene, Roche: Honoraria. Villacampa:AstraZeneca: Other: advisory role; Merck Sharp & Dohme: Honoraria. Abrisqueta:AbbVie: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Celgene: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Speakers Bureau. Bosch:Hoffmann-La Roche: Research Funding. Barba:Novartis, Celgene, Gilead, Pfizer, Amgen, Roche: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal